Abstract
Introduction: Decision-makers need to understand long-term outcomes to assess cost-effectiveness of new treatments. In the absence of long-term data from clinical trials, current practice to extrapolate overall survival (OS) is typically based on how well the model fits to the observed data without formally involving clinical experts to evaluate the plausibility of the results. At best, 1-2 clinicians may be asked to select the most 'realistic' model. Therefore, a more systematic and transparent process is needed to ensure clinical expertise is fully integrated in an unbiased manner. In the global multicenter ELIANA trial (NCT02435849) for CTL019 (chimeric antigen receptor T-cell (CAR-T therapy)), OS data has been reported, but only up to 1.5 years. In the absence of expert opinion regarding long-term OS, the predicted OS varies dramatically depending on the model used to extrapolate the observed data. The aim of this study was to estimate expected OS based on observed data from ELIANA and expert elicited OS rates at 2 years, 3 years, 4 years, and 5 years for patients with relapsed or refractory B-cell pediatric acute lymphoblastic leukemia (r/r/ pALL) treated with CTL019.
Methods: A prospective qualitative research study was performed incorporating semi-structured interviews, adapted from the SHeffield ELicitation Framework. Pediatric oncologists and hematologists experienced with CAR-T therapies were recruited. Experts and the study sponsor were not identified to one another. Relevant evidence regarding the patient population from the CTL019 trials, historical first-line pALL data, and historical data on r/r/ pALL for FDA approved interventions was summarized to provide a common basis for expert judgments. Facilitators guided experts through a web-based application for the elicitation of OS rates at 2, 3, 4, and 5 years for patients treated with CTL019 in the ELIANA trial. Experts were first asked to estimate upper and lower plausible limits and then the most likely actual OS value at each time point. Results from each expert were combined with the observed data from ELIANA using time-to-event parametric models (i.e. fractional polynomials) in a Bayesian framework that accounted for each expert's uncertainty in their estimates, which produced an overall distribution of OS over time. In addition, the model was fully extrapolated assuming patients followed general population mortality, adjusted for having had pALL (standard mortality rate=15.2 based on Armstrong 2016).
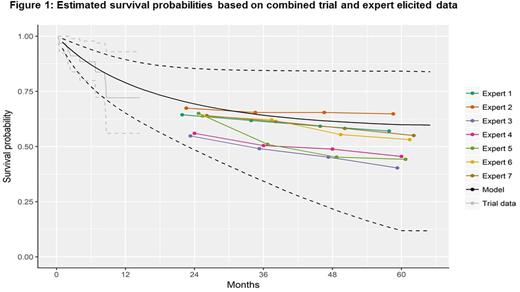
Results: Of the 19 clinical experts contacted, 7 participated in the final elicitation exercise. During a follow-up group meeting, experts agreed that the Gompertz model fit to the observed trial data reflected an appropriate consensus distribution for their estimates. When a Gompertz model was fit to the observed data (up to 1.5 years) and each expert's elicited data (24 to 60 months), the most likely values of OS for patients from the ELIANA trial treated with CTL019 at 2, 3, 4, and 5 years were estimated to be approximately 67.5% (Credible intervals (CrI): 49.5%, 83.1%), 61.1% (39.5%, 81.3%), 57.2% (31.5%, 80.7%), and 54.9% (24.5%, 80.5%) (Figure 1). In the fully extrapolated model, the 10-year OS is estimated to be 52.4% (CrI: 23.4%, 76.9%) with a median survival of approximately 13.4 years (CrI: 1.96, 35.5). Results were similar to OS rates from a phase I/II single-center study (NCT01626495) at 2 years (61%) and slightly higher at three years (52%).
Conclusions: Predictions based on observed data and expert elicitations suggest that more than half of the patients in the ELIANA trial treated with CLT019 will survive up to five years. This study provides an example of how expert opinion can be elicited and synthesized with observed survival data using a transparent and unbiased process, which captures expert uncertainty and ensures that projected long-term survival is clinical plausible.
Disclaimer: The study was sponsored by Novartis Pharmaceuticals Corporation
Cope: Precision Health Economics: Employment; Novartis Pharmaceuticals Corporation: Consultancy. Ayers: Precision Health Economics: Employment; Novartis Pharmaceuticals Corporation: Consultancy. Shih: Precision Health Economics: Employment; Novartis Pharmaceuticals Corporation: Consultancy. Zhang: Novartis Pharmaceuticals Corporation: Employment. Jansen: Novartis Pharmaceuticals Corporation: Consultancy; Precision Health Economics: Employment. Batt: Precision Health Economics: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy; Bayer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal